Listeria is a rod-shaped bacteria which naturally occurs in almost all environments – on plants and in the intestines of humans and animals. The Listeria monocytogenes species is characterized by its high degree of resistance.
Why is listeria dangerous?
Listeria monocytogenes can cause listeriosis in humans, a rare but serious form of food-borne infection which must be reported to the authorities.
In healthy adults the infection is generally harmless with sufferers experiencing flu-like symptoms. It can, however, cause severe illness or even death among risk groups such as the elderly, those who are immunocompromised or babies. Pregnant women are also particularly at risk as they can pass on the infection to their baby, leading, among other things, to premature or stillbirth.
If the food business operator can demonstrate by historical documentation that he uses the AVO protection concept without any gaps, has an effective HACCP-based food safety concept and that growth of L. monocytogenes in the corresponding products is not supported, then the product is automatically placed in Category 1.3.
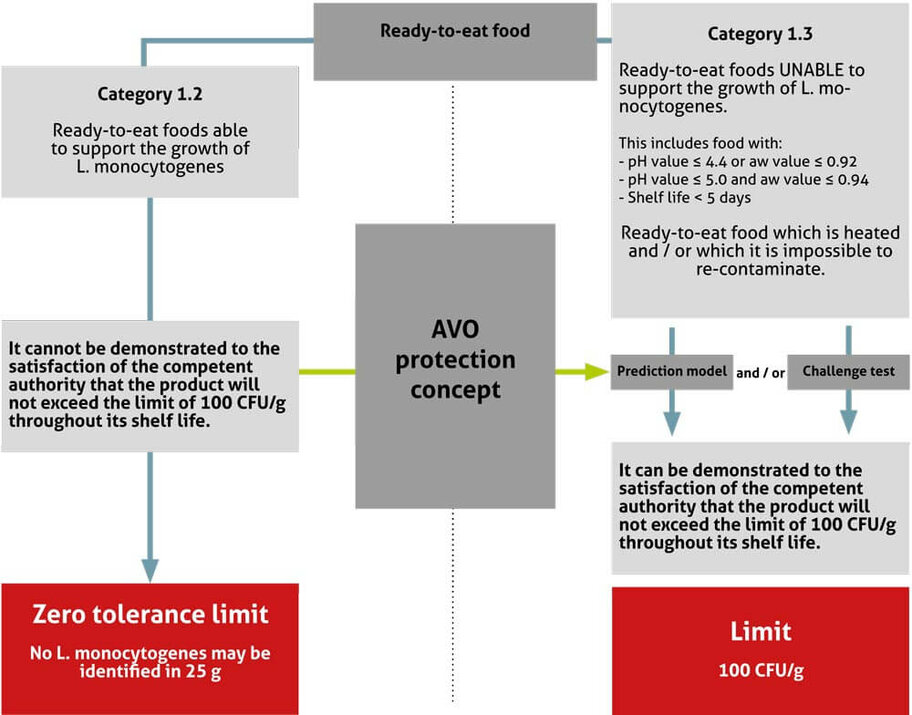
Growth of L. monocytogenes is primarily dependent on the following factors:
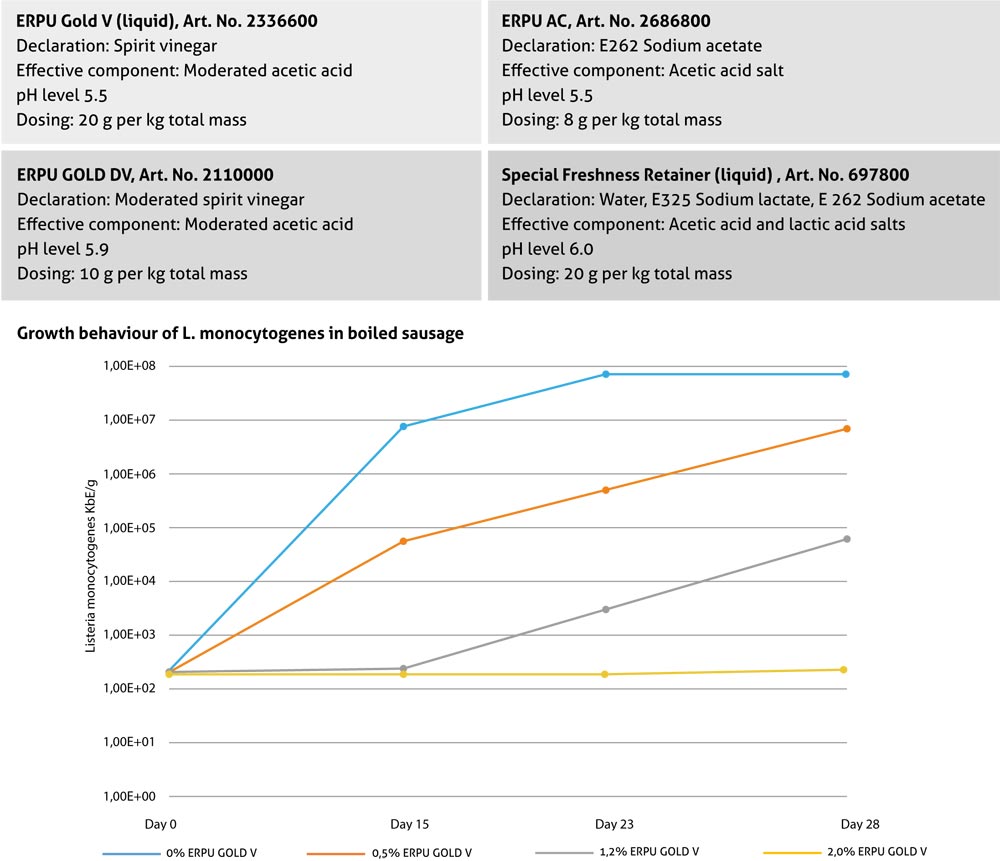
The AVO product range offers effective products to maintain freshness:
Defined, standardised addition of starter cultures provides an important contribution when manufacturing fermented meat products:
Protective cultures are:
The following modes of action are employed:
This test was carried out using a Teewurst sausage manufactured using the new AVO Meisterclub raw sausage protective culture Art. No. 1589500 (for 50 kg), AVO Meisterclub raw sausage protective culture Art. No. 1589600 (for 100 kg) and a standard ripening culture, in this case AVO Meisterclub starter cultures for spreadable raw sausage Art. No. 638300.
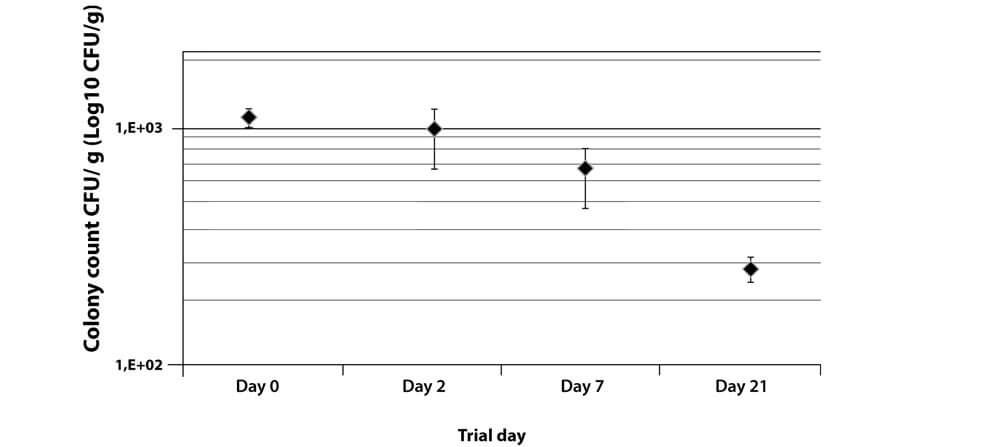
Can be used for any kind of raw sausage and in particular for products such as Teewurst, Pfefferbeisser, Mettendchen, Kohlwurst, etc.: